Easy Naming Ionic and Covalent Compounds Sheet
Why Do We Name Compounds?
Naming a compound gives us an easier way to discuss it in conversation. Just imagine having to say "aitch-gee-too-bee-ar-too dissolved in aitch-too-oh" and the like multiple times per conversation - it's much more natural to say "mercury bromide dissolved in water"! Naming a compound properly gives us this ability to talk about a compound naturally without losing any information about the compound.
Naming Covalent (Molecular) Compounds
- Recall that covalent compounds are those that involve more than one atom bonded together by the sharing of electrons. You'll know for certain that you are dealing with a molecular compound if only nonmetals are present.
- To name a covalent compound, you need the molecular formula, knowledge of the prefixes used for naming, and a way to look up the name of an element given its atomic symbol. With this information in hand, you can follow the naming scheme for covalent compounds:

phriot
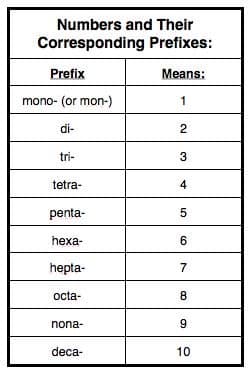
Steps to Naming Covalent Compounds
- First, identify the elements present.
- Second, look at the subscript of each element to determine which prefix to use. (If an element does not have a prefix, assume that the subscript is "1."
- Third, apply the above naming scheme. (Note: If the prefix of the first element would be "mono-", it is not needed.)
TIP!: Get used to what part of an element's name is the "root" early, because it's not always easy to tell by looking!

Naming Ionic Compounds
- Recall that ionic compounds consist of a positively charged cation and a negatively charged anion.
- Ions (of either variety) may contain either a single element or more than one element. (When an ion consists of more than one element, we refer to it as a "polyatomic ion.")
- To recognize an ionic compound, look for the presence of a metal or a known polyatomic ion - once you find one, you more than likely have an ionic compound.
- When we name an ionic compound, we do not use prefixes; instead, use one following naming schemes:
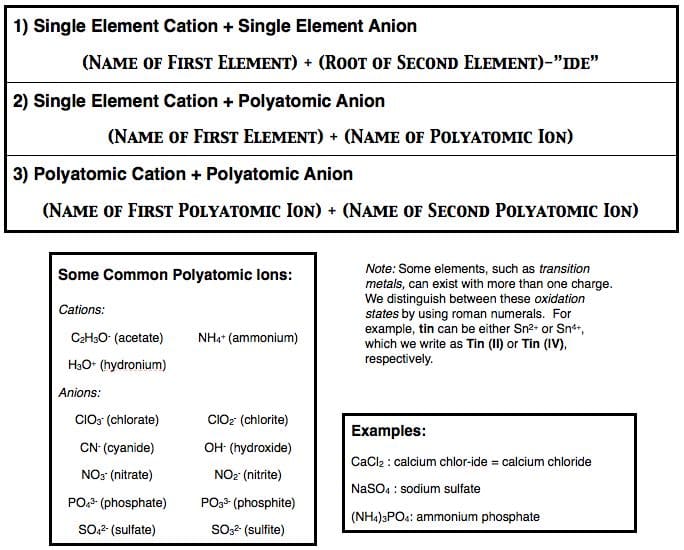
Test Your Knowledge!
For each question, choose the best answer. The answer key is below.
- Name the compound CO.
- Monocarbon Monoxide
- Carbon Monoxide
- Carbon Dioxide
- Name the compound NaCN.
- Sodium Cyanide
- Sodium Carbon Nitride
- Sodium Monocarbon Mononitride
Answer Key
- Carbon Monoxide
- Sodium Cyanide
Duff on September 19, 2019:
@MAbala when the element has 2 letters for its symbol the second is lower case (bromine is Br) but CN is the combination of carbon and nitrogen coming together to form the polyatomic ion cyanide which is why both the C and the N are capitalized
MAbala on September 09, 2019:
Scroll to Continue
Read More From Owlcation
Is there a rule which mandates the second letter in an abbreviation of any element in the periodic table to be lower-cased. Because in the quiz above, cyanide was written 'CN'
For Ex. Br for bromine with an uppercase 'B' and a lowercase 'r'.
Gavindhale on April 04, 2019:
the intulenticity of this site is very poorley accounted and I would like it to be removed as of April the 4th and Fortnite is the best game ever made in the history of the whole wide world
Fortnite on September 28, 2018:
Noooo...
Break trees for wood
Choder on October 03, 2017:
Thanks bon
Bob on February 16, 2017:
Maybe explain why each type of bond has a set of different naming rules
phriot (author) on January 05, 2017:
Hi Jared. It's been a while since I've looked at my Hubs, so I didn't realize that I missed a rule here. Typically, if there is only one atom of the first element in a covalent compound, we omit the "mono-/mon-."
Jared on December 14, 2016:
Hey phriot, I was just doing the quiz and ran into something that didn't make sense if we follow the rules you laid out. The rule said that for molecular compounds that only contained non-metals we use (# prefix) first compound + (# prefix) second compound, but in the quiz it wants carbon monoxide instead of monocarbon monoxide, and I'm just confused when the leading element gets the prefix, when it doesn't, and what rules there were surrounding that?
Harry on November 08, 2016:
some simple and hard quiz questions would be nice
rylie on October 04, 2016:
hi i'm a college student in South Dakota and I found this useful! more quiz questions would help! Thank you!
Liwayway Memije-Cruz from Bulacan, Philippines on February 24, 2015:
I am teaching General Chemistry to Hotel Management and Tourism Management students in one of the universities in Bulacan...I am using on line method to make lessons easier for them. I found your hubs very interesting, useful and simple enough to substantiate my lessons. Congratulations and more power.
phriot (author) on March 12, 2013:
Hi Key! I appreciate that you need help, but I'm not fluent in Spanish, so it would be difficult for me to communicate these concepts in that language. Good Luck!
key on March 12, 2013:
necesito ayuda
phriot (author) on February 26, 2013:
Thanks for the comment, Joe. The two question quiz I have here is the most I can do easily with this platform, but I'll work on either hosting my own and posting a link or providing a link to a good quiz in another location. I'm also hoping to have time soon to make a video where I work out an example or two live.
joe on February 06, 2013:
ya a legit quiz or two would be nice and some work-out examples would be amazing
phriot (author) on October 09, 2012:
I notice that I'm getting a lot more views on this hub lately. To those that are new readers, what could I do to make this hub better for you? Would you like a quiz to check your knowledge? More worked-out examples? Add some color to spice things up? Let me know!
aldridgewearprapart.blogspot.com
Source: https://owlcation.com/stem/Naming-Chemical-Compounds
0 Response to "Easy Naming Ionic and Covalent Compounds Sheet"
Post a Comment